Abstract
Background: Chimeric antigen receptor (CAR) T-cell therapy has shown promising responses in various hematologic malignancies. Cytopenias including anemia, neutropenia, and thrombocytopenia are common and manageable toxicities in the first 90 days after CAR T-cell therapy. Factors contributing to hematopoietic recovery following CAR T-cell therapy have not been well studied.
Objective: Characterize the cytopenias, potential associated risk factors, incidence and management of infections, utilization of granulocyte-colony stimulating factor (G-CSF), thrombopoietin receptor (TPO) agonists, stem cell boosts, and immunoglobulin (IVIG) in the first 90 days after CAR T-cell infusion.
Methods: This retrospective, single center chart review included 49 patients with various hematologic malignancies (non-Hodgkin lymphoma, acute lymphoblastic leukemia, multiple myeloma) treated with CAR T-cell therapy between March 2019 and July 2022. White blood cell count, absolute lymphocyte count, absolute neutrophil count, hemoglobin, platelet counts, and inflammatory markers were recorded at apheresis, lymphodepletion (day -5), day 0, +7, +14, +21, +30, +60, and +90. Cytopenias were documented using CTCAE grading system. Hypogammaglobulinemia (IgG<400 mg/dL) and infections by day +30 and day +90 were evaluated. Administration of GCSF, TPO-agonist, and IVIG up to day +90 were recorded. Recovery of immune repertoire using bone marrow biopsy and immunodeficiency panel results were included when available. Responses to CAR T were documented using disease specific disease response criteria. Cytokine release syndrome (CRS) and immune effector cell associated neurologic syndrome (ICANS) were graded using the ASTCT consensus criteria. The data was censored at date of progression, death or day +90 post CAR T-cell infusion.
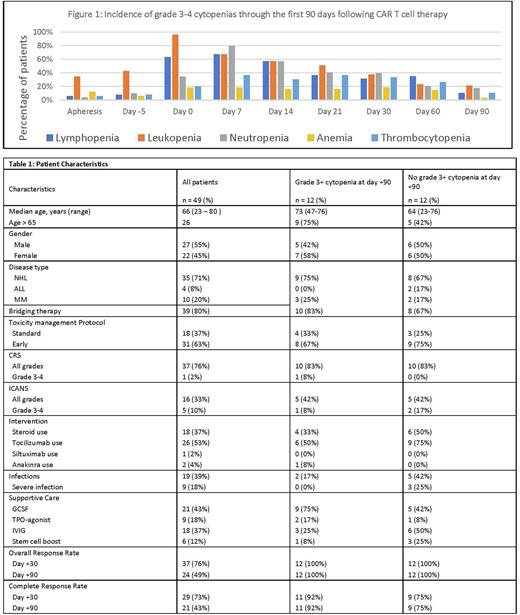
Results: Forty-nine patients received CAR T-cell therapy including axicabtagene ciloleucel (n = 6), tisagenlecleucel (n = 13), brexucabtagene autoleucel (n = 9), lisocabtagene maraleucel (n = 11), idecabtagene vicleucel (n = 10) from March 2019 to March 2022. Patient characteristics for all patients and those with and without grade 3-4 cytopenias at day +90 are seen in Table 1. The incidences of grade 3-4 lymphopenia, neutropenia, anemia, and thrombocytopenia at day 0 were 96%, 35%, 18%, and 20%; at day 30 the incidences were 38%, 40%, 19%, and 33%; at day +90 the incidences were 21%, 18%, 4%, and 11%, respectively with further characterization in Figure 1. Day +90 overall response rate and complete response rates were 49% and 43%, respectively with no significant differences between patients with and without cytopenias on day+90. Any grade CRS and ICANS were seen in 76% and 33% of the patients with grade 3+ CRS and ICANS seen in 2% and 10% of the patients, respectively. No significant differences were observed in the incidence of cytopenias in patients with any grades or grade 3+ CRS and ICANS. Infections occurred in 39% of patients and were severe, requiring hospitalization and intravenous treatment in 14%. The median time to infection was 8 days post infusion with 63% of infections having a bacterial source and 52% a viral source. None of the patients with cytopenias developed underlying myelodysplastic syndrome post CAR T. No cytopenia or infection related deaths were noted. Per physician discretion, GCSF was administered to 21 patients with median time to initiation of 23 days (range 14-140 days) and median duration of use of 33 days, with four patients having ongoing use at the time of last follow up. TPO-agonists were administered to nine patients. Eighteen patients received IVIG. Six patients received a stem cell boost due to prolonged cytopenia at a median of 54 days post CAR T-cell infusion and led to successful recovery of cytopenias. Further statistical analysis is under consideration to assess patient and disease related factors that may contribute to delayed hematopoietic recovery and associated complications of infections, bleeding and transfusions and will be available for the meeting.
Conclusion: Cytopenias commonly persist through day +90 days post CAR T-cell therapy. Infections are common but manageable. The use of GCSF, TPO agonists, and IVIG appeared safe and did not worsen CRS or ICANS.
Disclosures
Hashmi:JANSSEN: Consultancy; KARYOPHARM: Speakers Bureau; GSK: Speakers Bureau; Sanofi: Consultancy, Speakers Bureau; BMS: Consultancy.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal